This was an awesome day. I was actually pretty nervous leading up to it, but after the first attempt it was not too bad. I just missed the 165 pound weight class by three pounds so they bumped me to 181. Next time I’ll keep a closer eye on my weight and try to go down to the 165 class to be more competitive. My goal was just to go 9-for-9 on my attempts and I did. I put up a 300 squat, 235 bench press, and 410 deadlift for a total of 945. On my last squat and deadlift I definitely felt like I had more in the tank so I can actually lift more than what I put up. But I am happy with the results and proud of finally getting up there to compete. Nothing makes you more focused and concentrated on training than having a competition on the horizon. I will for sure be doing more of these in the near future. For now, it’s back to training and trying to get bigger and stronger over the winter.
Saturated vs Unsaturated Fats
Ever wonder what the difference is between saturated and unsaturated fats? Are they healthy or unhealthy? Is one better than the other? What does omega-3 and omega-6 really mean? Well wonder no more my friends, in this post I am going to explain what saturated and unsaturated fats are and which you should be including in your diet.
What is Fat?
Fat refers to the nutrients known as lipids. This family of nutrient includes triglycerides, phospholipids, and sterols. Triglycerides are the most abundant lipid both in food and in the body, making up 95% of the total fat. Saturated and unsaturated fats are within the triglyceride umbrella so let’s define what they are.
Triglycerides
Triglycerides refer to a molecule of glycerol (an alchol) and three fatty acids that make up a chemical chain:
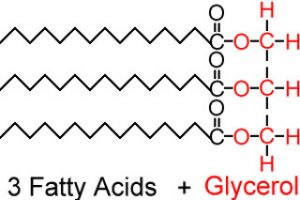
Chemistry, man.
So every single triglyceride has the same basic makeup. They always have a glycerol and three fatty acids. The glycerol never changes, but the makeup of the fatty acids does.
Fatty Acids
Fatty acids are composed of a chain of carbon and hydrogen atoms with an acid group (COOH) at one end of the molecule and a methyl group (CH3) at the other end. The fatty acids of a triglyceride molecule vary in length and in the number and location of their double bonds. Most fatty acids in our diet are 18 carbons long.
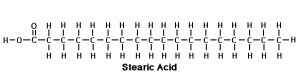
Stearic acid is an 18-carbon saturated fatty acid.
All you really need to know when it comes to these fatty acids is whether they are saturated or unsaturated. When a fat is saturated, it simply means that all the hydrogen atoms in the molecule are filled. If you look at the picture above, you see that all the chemical bonds are full. Simple right? All animal products contain saturated fat to some degree, and only a couple plant products contain them (coconut oil, palm oil, palm kernel oil). Now let’s talk about unsaturated fats, which look like this:
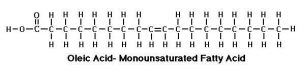
Oleic acid is an example of a monounsaturated fat.
Unsaturated fats occur when there are a lack of hydrogen atoms in the chain. When this happens, the carbon atoms that would have bonded to hydrogen form a double bond and thus the molecule is not completely saturated anymore. The point where this double bond occurs is called the point of unsaturation. The number of points of unsaturation in a fatty acid determine its name. One double bond is a monounsaturated fatty acid; two or more is polyunsaturated.
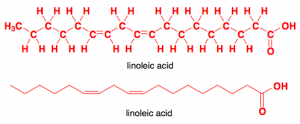
Linoleic acid is a ployunsaturated fatty acid.
When it comes to polyunsaturated fats, the location of the bond is important to know as well. Chemists identify them based on how far away the point of unsaturation is from the CH3 end of the molecule. An omega-3 fatty acid has its double bond three carbons away from the end, omega-6 is six away, and omega-9 is nine carbons away from the CH3. Unsaturated fats are mainly found in plant based products such as olive oil, vegetable oil, seeds, nuts, and are also found in fish. So now that you’re super confused with all this chemistry I’ve thrown at you, let’s recap: triglycerides are the most abundant of dietary fats and are made up of a glycerol and three fatty acids. The makeup of the fatty acids varies, but they are made up of a long chain of carbon atoms bonded to hydrogen atoms with an acid group at one end and a methyl group at the other. If a fatty acid has all the carbons bonded to hydrogen, it is saturated. If there is an open space where hydrogen is missing, the carbons bond to each other and the molecule is unsaturated.
What does it all mean?
By this point you may be wondering what the point of all that chemistry was. Well I’ll tell you: it was to provide you with a basic understanding of how these fatty acids are named. Now that you are armed with that knowledge we can discuss which to consume and which to avoid. It is best to avoid saturated fats and consume unsaturated fats. So try to limit (or eliminate entirely) your intake of whole dairy products, fatty meats, and junk food. The American Heart Association recommends limiting saturated fat to 13 grams or less per day. Instead focus on including seeds, nuts, and plant based oils in your diet.
